Why Do Nonmetals Have High Ionization Energy
They have more electrons in the valence shell compared to. Do nonmetals have a low first ionization energy.

Explained Why Do Non Metals Have High Ionization Energy
They are poor conductors.

. Why do nonmetals have higher ionization energies. Stay tuned with BYJUS to learn more about other concepts such as ionization energy. This tendency makes them have more electrons in the valence shell and thus have more ionization energy values than the metals.
Because non-metals have to gain electrons so they have more valence electrons than metals. Why do non-metals have high ionization energy. Nonmetals have a greater electron affinity than metals because of their atomic structures.
Nonmetals have higher ionization energy than metals because they need to add electrons. Therefore it requires more energy to remove them. Do Nonmetals have High Ionization Energy They have to gain electrons from non-metals because they have more valence electrons than they have metals.
They have small atomic radii and large ionic radii. Why do nonmetals have a higher ionization energy than metals. Non-metals have high ionization energy values than metals because non-metals have the tendency to gain electrons.
So the first ionization energy for a nonmetal is much greater than that of an alkali metal so nonmetals do not lose electrons in an ionic bond but instead gain one or more electrons and form anions. Why do nonmetals have high ionization energy values. Non-metals are found on the upper right side of the period table.
Non-metals have high ionization energy high electron affinity and high electronegativity. Unlike metals that are trying to lose electrons they need more energy to gain them. A non-metal receives an electron when a metal lacks an electron.
First nonmetals have more valence electrons than metals do thus it is easier for the nonmetals to gain electrons to fulfill a stable octet and secondly the valence electron shell is closer to the nucleus thus it is harder to. Was this answer helpful. To obtain Noble Gas configurations non-metals tend to gain electrons.
The atoms of nonmetals have smaller radii therefore the positively charged nucleus has a greater attraction for the valence electrons. Therefore eliminating them takes more resources. What makes an atom have a low ionization energy.
When a metal loses an electron a nonmetal gains an electron. - Answers Non metals have high ionisation energies since they tend to gain electrons.

Solved In General Do Metals Or Nonmetals From The Same Period Have Higher Ionization Energies Why A Metals Have Higher Ionization Energies Because They Usually Have More Protons Than Nonmetals B Nonmetals Have

Do Nonmetals Have High Ionization Energy
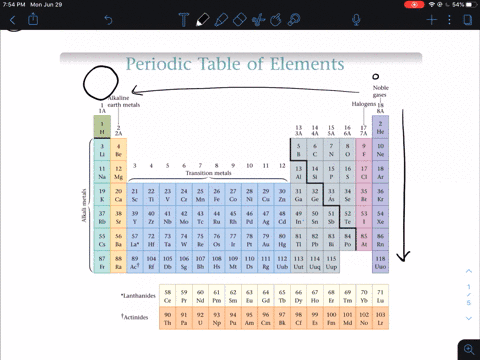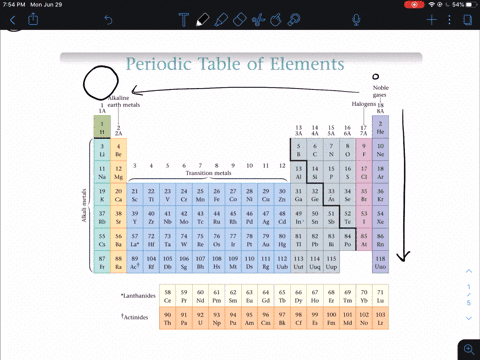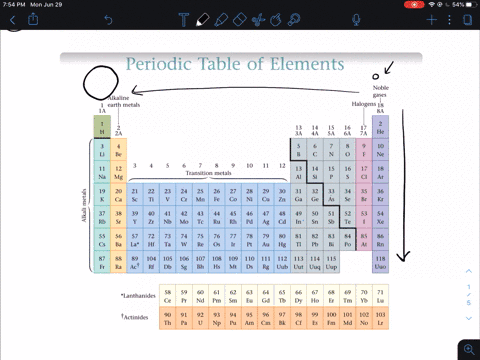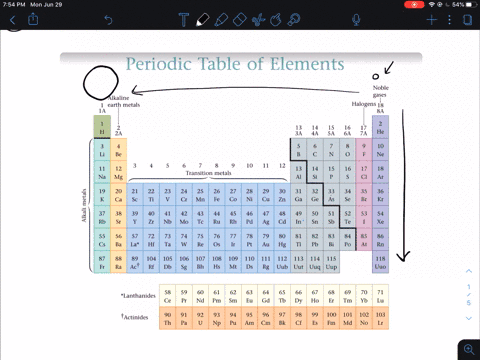
Solved In General Do Metals Or Nonmetals From The Same Period Have Higher Ionization Energies Why A Metals Have Higher Ionization Energies Because They Usually Have More Protons Than Nonmetals B Nonmetals Have
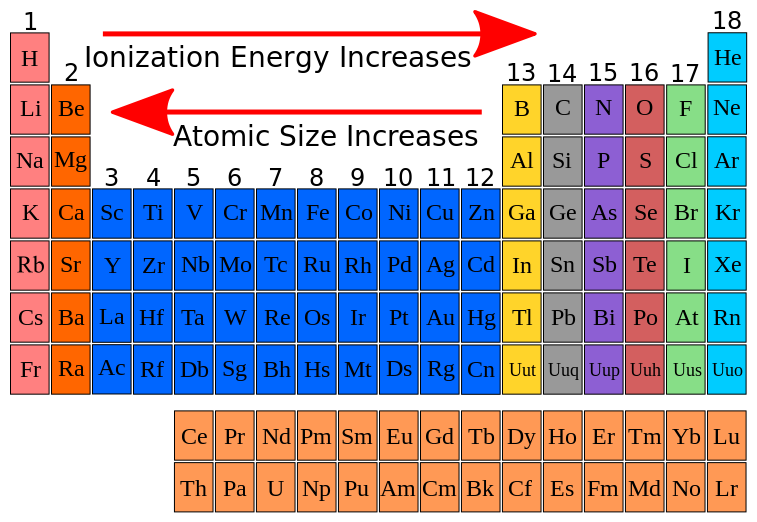
Why Is The First Ionization Energy Of A Nonmetal Significantly Higher Than That Of An Alkali Metal Socratic
No comments for "Why Do Nonmetals Have High Ionization Energy"
Post a Comment